One of the most-cited concerns about all-electric cars — battery capacity — will soon become moot thanks to a new nanomaterial capable of tripling the capacity and extending the service life of lithium-ion batteries, according to a new study published in the Journal of Alloys and Compounds.
The inherent limits of graphite in all-electric batteries
Scientists of the National University of Science and Technology “MISIS” (NUST MISIS), part of an international team of researchers, managed to increase the capacity and extend the service life of lithium-ion batteries. According to the researchers, they have synthesized a new nanomaterial that can replace low-efficiency graphite used in lithium-ion batteries today.
Lithium-ion batteries are widely used for household appliances from smartphones to electric vehicles. The charge-discharge cycle in such battery is provided by the movement of lithium ions between two electrodes—from a negatively charged anode to a positively charged cathode.
The scope of application of lithium-ion batteries is constantly expanding, but at the same time, according to the scientists, their capacity is still limited by the properties of graphite—the main anode material. Scientists from NUST MISIS managed to obtain a new material for anodes that can provide a significant increase in capacity and extend battery service life.
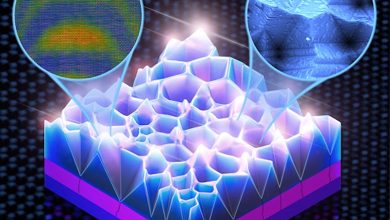
“Porous nanostructured microspheres with the composition Cu0.4Zn0.6Fe2O4, that we have extracted, used as anode material provide three times higher capacity than the batteries existing on market. Besides, it allows for an increase in the number of charge-discharge cycles by 5 times compared to other promising alternatives to graphite. This improvement is achieved due to a synergistic effect with a combination of a special nanostructure and the composition of used elements,” Evgeny Kolesnikov, an assistant at the Department of Functional Nanosystems and High-Temperature Materials, NUST MISIS said.

Applied scientific advancements can soften all-electric skeptics
The final material was synthesized via a one-step process — with no intermediate stages, thanks to the spray-pyrolysis method. According to the scientists, an aqueous solution containing ions of special metals is converted into fog using ultrasound, with water subsequently evaporated at temperatures reaching 2,192°F (1,200°C) as the original metal salts decompose.
The output is micron or submicron spheres with the specific porosity needed to function in a lithium-ion system — which is extracted from the solution.
Further electrochemical studies of the new nanomaterial synthesized by NUST MISIS were executed by scientists of Seoul National University of Science and Technology, the SRM Institute of Science and Technology (in India), and the Norwegian University of Science and Technology.
Like it or not, we’re entering an all-electric revolution — with heavy-hitting newcomers like Tesla leading the way as GM, Audi, and many other brands working to move away from fossil fuel vehicles in the next 10 to 30 years. But, while smartphones and other common electronics will phase out old batteries with fewer bumps, scientists will need to find crucial advancements like this new nanomaterial to soften concerns EV skeptics have about the next generation of personal vehicles.