A new study by a team at the University of Chicago, published in the October 14, 2021 issue of the journal Nature Physics, reports on the creation of a new phase of ice called “superionic ice”. Turns out, the ice that tinkles in our glasses of Coke, known as Ih, is actually just one of at least 19 different phases of ice.
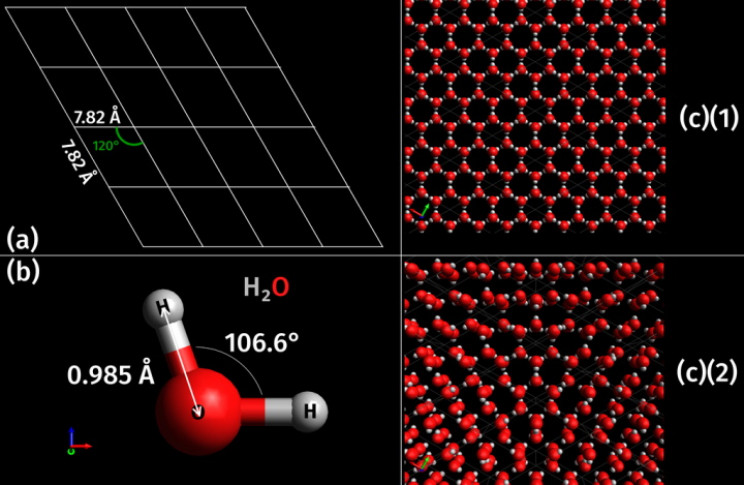
Formed from water, ice is comprised of only hydrogen and oxygen atoms in the famous H2O configuration of two hydrogen atoms attached to one oxygen atom.
One intriguing idea is that ice may become superionic when heated at very high temperatures and pressures. This exotic state would contain liquid-like hydrogen ions moving within a solid lattice of oxygen.
Superionic ice was first predicted in 1988, and since then a number of research groups have used simulation and static compression techniques to try and study this phase of ice.
The first experimental evidence for superionic water ice came from a 2018 study by scientists from the Lawrence Livermore National Laboratory (LLNL), UC Berkeley, and the University of Rochester. They first sandwiched a droplet of water between two diamonds that functioned like miniature anvils, squeezing the droplet with 2.5 GPa of pressure (25 thousand atmospheres). This “pre-compressed” water into the room-temperature ice VII, a cubic crystalline form of ice.
The team then shifted to the University of Rochester’s Laboratory for Laser Energetics where they bombarded one of the diamonds with up to six intense beams of UV light. This launched strong shock waves of several hundred GPa into the sample, to compress and heat the ice at the same time. The result verified the existence of super-ionic ice but was only able to create it for a few nanoseconds before it melted away – not long enough to measure its properties.
In a more recent study, conducted in 2019, the team was able to create a more stable form of the ice by squeezing a water droplet with a 0.2-carat diamond anvil and blasting it with a laser, pressurizing the droplet to 3.5 million times Earth’s atmospheric pressure at temperatures hotter than the surface of the sun. The ice was the eighteenth form to be discovered, and so was named Ice XVIII (“Ice 18”).
In Ice XVIII, oxygen atoms in the droplet took up stationary positions, while the hydrogen atoms, which were stripped of their electrons thus turning them into positively charged ions, were free to flow throughout the ice, acting like a fluid. The free-flowing ions blocked all light from passing through the ice, making the ice black in color.
The work published in 2021 by the team in Chicago used similar methods to elucidate what may be another phase of superionic ice. They squeezed water droplets in a diamond anvil to pressures of 20 GPa, and shoot lasers through the diamonds to heat the sample up. Finally, they sent a beam of X-rays through the sample, and pieced together the arrangement of the atoms inside the superionic ice by observing how the X-rays scattered off the sample.
The positively-charged, free-flowing hydrogen ions in superionic ice also create a magnetic field, and this is highly interesting to scientists because many icy bodies in our solar system, such as Neptune, Uranus, and Jupiter’s moons, Europa, Io, and Ganymede, have magnetic fields. Scientists are now wondering if those magnetic fields are caused by the presence of superionic ice in the cores of those bodies.
This question is vital since a planet’s magnetic field, or magnetosphere, is what prevents dangerous cosmic rays and UV radiation from reaching a planet’s surface and obliterating all life. If superionic ice is common in the cores of planets outside our solar system, that would make the possibility of life on other planets that much more likely.
Different phases of ice
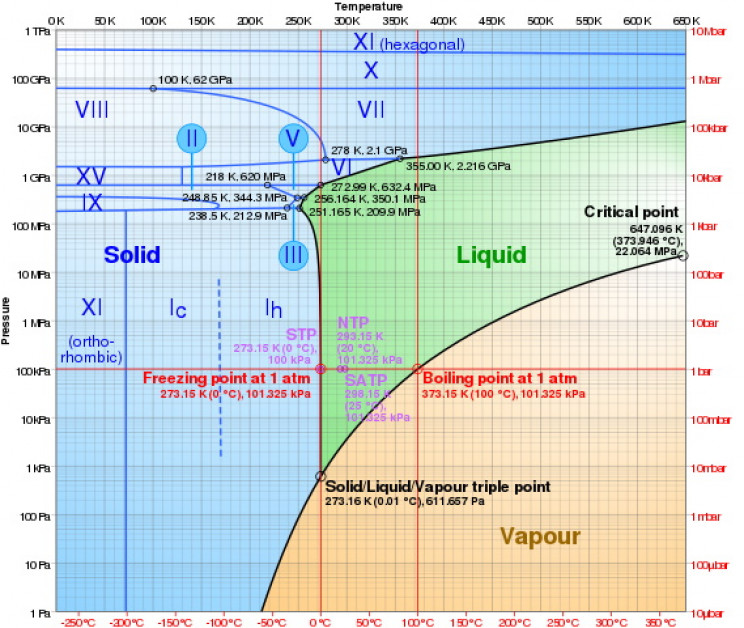
The different phases of ice are differentiated by their crystalline structure, or the ordering of their protons, and also by their density. The most common ice on Earth, Ih, occurs when liquid water is cooled to below 32 ° F, 0 ° C, or 273.15 ° K at standard atmospheric pressure.
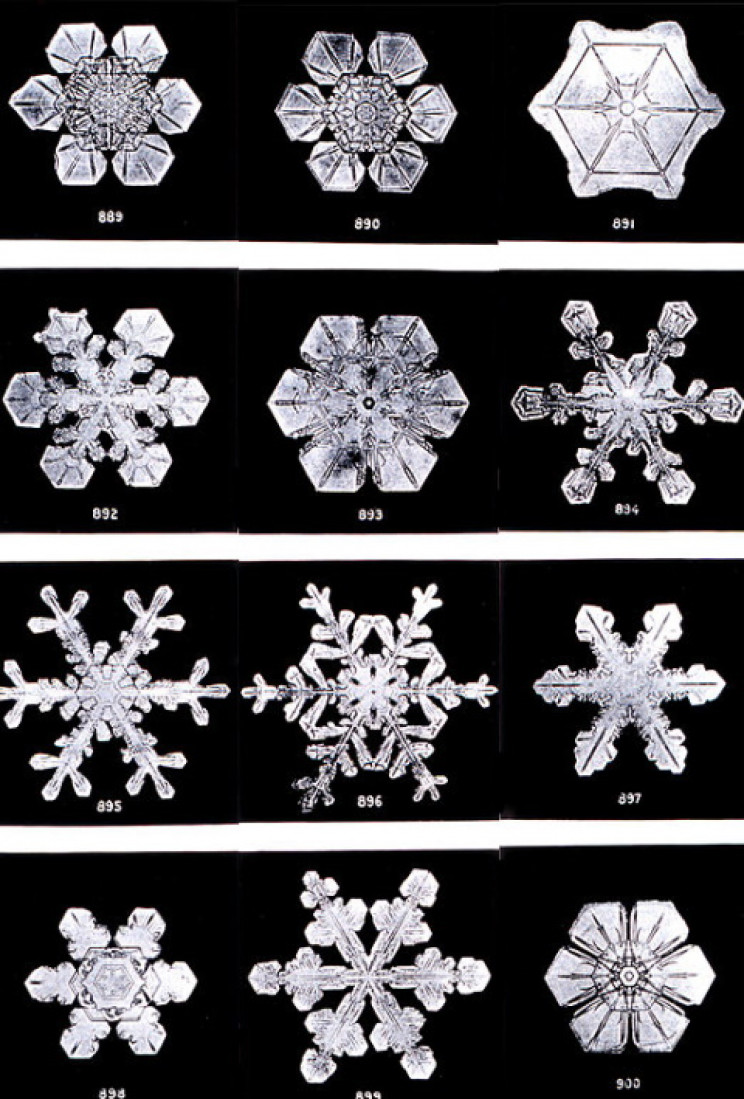
Ih has a hexagonal, or six-sided, crystalline structure, and that structure is mirrored in the infinite variety of six-sided snowflakes. In Ih the oxygen atoms take on the hexagonal shape, while the hydrogen atoms take up positions around them. The hydrogen atoms are termed “disordered”.
The next most common phase of ice on Earth, Icec, has atoms arranged in a diamond structure. It is formed at temperatures between 130 K (-226 °F) and 220 K (-64 °F), and can exist up to 240 K (-28 °F), and can be found in Earth’s upper atmosphere, where it plays a role at very low temperatures in the nucleation of ice.
Ice II has a rhombohedral crystalline structure, with six faces which are rhombi, and it is formed by compressing Ih at between 190 K (-118 °F) and 210 K (-82 °F). Ice III has a tetragonal crystalline structure consisting of three axes at right angles, two of which are equal, and it is formed by cooling Ih down to 250 K (-370 °F) at a pressure of 300 MPa. (1 Megapascal (MPa) is equal to 145.04 pounds psi.)
To create Ice IV, which has a rhombohedral structure, you need a nucleating agent which affects the temperature at which crystallization occurs. Ice IV is the lowest, high-pressure ice phase at room temperature and has been spectroscopically found in diamond inclusions. It requires a pressure of 810 Mpa. Ice V is formed by cooling water to 253 K (-4.27 °F) at 500 MPa, and it has a complicated crystalline structure, including 4-membered, 5-membered, 6-membered, and 8-membered rings and a total of 28 molecules in the unit cell.
Ice VI has a tetragonal crystalline structure, and is formed at temperatures up to 355 K (179.33 ° F) and a pressure of 1.1 GPa. The oxygen atoms in Ice VII have a cubic structure. This ice is unique in that it remains stable even at very high pressures, exceeding 30,000 atmospheres (3 gigapascals). In 2018, researchers at the University of Nevada found the first samples of naturally occurring Ice VII on earth, inside diamonds.
In Ice VIII, which is formed by cooling Ice-VII to below 278 K (40.73 °F) at around 2.1 GPa, the hydrogen atoms assume fixed positions.
Ice IX, which was discovered in 1968, has a tetragonal structure, and is formed from ice-3 by cooling it to between 208 K (-85.27 °F) to 165 K (-163 °F) with pressures between 200 MPa and 400 MPa. The atoms in Ice X are proton-ordered, symmetric, and form at around 60 to 70 GPa. Ice X is also thought to be stable to very high temperatures.
Ice-11, which was discovered in 1996, is the proton-ordered phase of ordinary ice, and features aligned water dipoles. It can be synthesized under laboratory conditions at temperatures of around 72 K (-330 °F) and it is ferroelectric, which means that its atoms can be spontaneously polarized.
Ice XII has a tetragonal structure. Pure ice XII can be created from ice Ih at 77 K (−196.2 °C; −321.1 °F) using rapid compression (0.81-1.00 GPa/min) or by warming high-density amorphous ice at pressures between 810 and 1600 MPa.
Ice XIII, which was discovered in 2006, is a proton-ordered form of Ice V. It has a monoclinic crystalline structure with three unequal axes of which one is at right angles to the other two. It is formed by doping water with HCL (at a ratio of around one molecule to every 5000 molecules of water) and cooling to below 130 K (-226 °F) at 500 MPa.
Ice XIV, which was discovered that same year, has an orthorhombic structure and is formed at temperatures below 118 K (-247 °F) at 1.2 GPa. It is the proton-ordered form of ice XII.
Ice XV is a proton-ordered form of Ice VI and is formed by cooling water to around 130 K (-226 ° F) at pressures of 0.8 to 1.5 GPa. Ice XVI is the least dense experimentally obtained crystalline form of water, while Ice XVII, also known as square ice, was discovered in 2014. It forms at room temperature when water is squeezed between two layers of graphene at more than 10,000 atmospheres of pressure. Graphene is a form of carbon that consists of a single layer of atoms arranged in a two-dimensional honeycomb lattice nanostructure.
The most recently discovered phase of ice is Ice XIX, described in 2021 by a team at the University of Innsbruck and verified by researchers in Japan. The newly identified form of ice is a hydrogen-ordered form of VI, which has a random pattern of hydrogen atoms. According to lead researcher Thomas Loerting, “Ice VI, ice XV, and ice XIX are all very similar in terms of density [because] they share the same kind of network of oxygen atoms. But they differ in terms of the positions of hydrogen atoms.”
Is life imitating art?

In 1963, famed writer Kurt Vonnegut published a novel entitled Cat’s Cradle. It is a strange amalgam of science, technology, the cold war, and religion. It concerns a writer who travels to the hometown of a fictional Nobel Prize winner physicist named Felix Hoenikker to determine what Hoenikker was doing on the day the U.S. dropped the atom bomb on Hiroshima, August 6, 1945.
The writer soon learns from Hoenikker’s three adult children that the famous scientist had been playing the string game “Cat’s Cradle” on that fateful day. The writer also learns that Hoenikker has created a mysterious substance called Ice-9, which is a phase of ice that remains solid at room temperature and won’t melt until the temperature reaches 114.4 °F.
Another unfortunate property of Ice-9 is that it acts as a seed crystal, snapping any water molecules it touches into the Ice-9 configuration. This would turn all water on Earth into a solid, thus ending all life.
One of the book’s characters, Dr. Breed, explains to the writer: “There are several ways … in which certain liquids can crystallize — can freeze — several ways in which their atoms can stack and lock in an orderly, rigid way.” Breed invites the writer to ” … think of the several ways in which cannonballs might be stacked on a courthouse lawn, of the several ways in which oranges might be packed into a crate.”
Breed says, ” … the pattern of the bottom layers of cannonballs or of oranges determined how each subsequent layer would stack and lock. ‘The bottom layer is the seed of how every cannonball or every orange that comes after is going to behave, even to an infinite number of cannonballs or oranges.'”
The real Ice-9 wasn’t discovered until 1968, five years after Vonnegut’s book was published. Gratefully, it doesn’t have any of the pernicious attributes of its fictional counterpart, and we’re not going to tell you what happens in the book if the fictional Ice-9 ends all life on Earth, but it does make you wonder about new phases of ice yet to be discovered.