Lead Image: Researchers have developed an ingestible capsule called FLASH (Fluid-wicking Active Stimulation and Hormone modulation) that can electronically stimulate the hunger-regulating hormone ghrelin in pigs. Inspired by the fluid-wicking skin of the Australian thorny devil lizard, the capsule emits electronic signals and moves through the body, eventually being excreted. This proof-of-concept study demonstrates the potential of ingestible electroceuticals for treating gastrointestinal, neuropsychiatric, and metabolic disorders. The team plans to continue researching for human application and explore treatment options for eating disorders and metabolic diseases. Credit: Giancarlo Traverso (GT Reel Productions)
The non-invasive FLASH system, inspired by lizard skin, electronically stimulates key hunger hormone.
Scientists have developed an ingestible capsule called FLASH that can electronically stimulate hunger-regulating hormones, offering a potential treatment for gastrointestinal, neuropsychiatric, and metabolic disorders.
Nature is the greatest teacher. A bizarre-looking lizard with intimidating spikes covering its body helped a team of investigators from Brigham and Women’s Hospital, a founding member of the Mass General Brigham healthcare system, Massachusetts Institute of Technology, and New York University, develop an innovative ingestible capsule that can modify ghrelin, a hunger-regulating hormone, in pigs. Their results, published today (April 26) in the journal Science Robotics, showed for the first time that the ingestible electronic fluid-wicking capsule for active stimulation and hormone modulation (FLASH) can be ingested to modulate gastrointestinal hormones through electrical stimulation of the stomach and safely excreted without side effects. The new system has potential applications for treating certain gastrointestinal, neuropsychiatric, and metabolic disorders.
“Our lab strives to develop systems that will make it easier and more accessible for patients to receive therapies,” said corresponding author Giovanni Traverso, MB, BChir, PhD, a gastroenterologist in the Brigham’s Division of Gastroenterology, Hepatology, and Endoscopy. “This is an exciting proof-of-concept study and a feat of fundamental research and engineering demonstrating the potential of ingestible electroceuticals.”
“An ingestible pill that contains electronics instead of chemicals or drugs is very promising,” said co-first author Khalil Ramadi, PhD, an assistant professor at New York University and a research affiliate at Brigham and Women’s Hospital. “It provides a way to deliver targeted electrical pulses to specific cells in the gut in a way that can regulate levels of neural hormones in the body.”
Here the authors describe the development and application of a new ingestible electroceutical capable of supporting the stimulation of ghrelin release. Credit: Giancarlo Traverso (GT Reel Productions)
The team previously observed that patients with gastroparesis, a disorder that slows or stops the movement of food from the stomach to the small intestine, who received gastric pacemakers, which operate using electrical stimulation, seemed to feel better in a manner disproportionate to the improvements in motility. This led them to investigate if the electrical stimulation was inducing a neurohormonal effect. They found that electrical stimulation supported the production of ghrelin, a hormone associated with regulating hunger and eating. After an investigation of the underlying mechanisms of this, the team found that the response was mediated by the vagus nerve, the body’s longest autonomic nervous system nerve that connects the brain and gut.
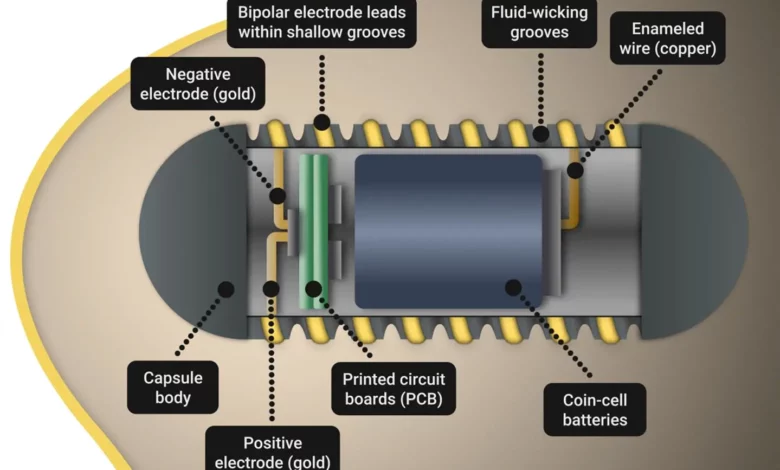
To stimulate the release of this important hormone, the team set out to create an ingestible capsule, known as an electroceutical, that could emit electronic signals and would move through the body, eventually being excreted. Electroceuticals are devices that use electric signals to treat various conditions. Because there is fluid in the stomach that can interfere with an electroceutical system, the investigators looked to nature for ways to wick away fluid and allow the system to still have good contact with stomach tissue.
That’s where the Australian lizard Moloch horridus, or “thorny devil” comes in; its fluid-wicking skin allows it to better absorb water in the arid regions where it lives. The team mimicked this lizard skin feature in the creation of its surface-treated hydrophilic capsule design, and created grooves sized to promote fluid wicking while retaining the capacity to hold an electrode to support stimulation.
In this study, the team endoscopically stimulated the inner surface of pig stomachs for twenty minutes and found that ghrelin increased in those with intact vagus nerves. The point of contact between the capsule and the stomach tissue was analyzed via CT scans. The FLASH system, which folds up electronics and a battery source inside of the capsule, was shown to reproducibly increase levels of ghrelin in pigs.
The team plans to continue this research for translational human application and is looking at how the approach might work in other areas of the body. Next, they plan to investigate how FLASH and similar ingestible electroceuticals could be used to treat eating disorders and metabolic diseases.
“This development provides many new avenues for research into the complex interconnections between the brain and gut and for furthering the use of electroceuticals as a clinical intervention,” said co-first author James McRae, a PhD Candidate at the Massachusetts Institute of Technology’s Department of Mechanical Engineering.
“The potential to modulate hormones using ingestible electroceuticals is potentially transformative because it does not require new drugs,” said Traverso. “Instead, it works alongside our physiological systems for the benefit of the person.”
Reference: “Bioinspired, fluid-wicking, ingestible electroceutical capsules for hunger-regulating hormone modulation” by Ramadi, K. et al., 26 April 2023, Science Robotics.
DOI: 10.1126/scirobotics.ade9676
Funding: This study was supported in part by a grant from Novo Nordisk, at the Massachusetts Institute of Technology (MIT) , the MIT Koch Institute’s Support (core) Grant from the National Cancer Institute, the National Institute for Diabetes and Digestive and Kidney Diseases, the Division of Engineering at New York University Abu Dhabi, a National Science Foundation graduate research fellowship and the Karl Van Tassel Career Development Professorship and the Department of Mechanical Engineering at MIT.
Disclosures: Khalil B. Ramadi, McRae, and Traverso are co-inventors on a patent application (No. 63/092,016) encompassing the work described.