AMD’s Ryzen 5000 Desktop CPUs have been introduced to the market and we are loving them. Recently, AMD officially introduced its, AGESA 1.2.0.1 BIOS Firmware which comes with a range of bug fixes and improvements for Ryzen 5000 Desktop CPUs. for the 500-series motherboards, the OEMs will soon be releasing the BIOS.
According to AMD, the first public BIOSes for Ryzen 5000 Desktop CPUs are rolling out now. AMD themselves confirm the following general fixes and improvements:
- Fix: False SMART errors on Hynix NVMe
- Fix: Intermittent SSD detection for M.2 SATA devices
- Improve L3$ bandwidth in AIDA64
- Improve stability if the user disables cores on 5600X/5800X with AMD Ryzen Master
AGESA 1.2.0.1 now arriving in public BIOSes for Ryzen 5000 Series!
1) Fix: False SMART errors on Hynix NVMe
2) Fix: Intermittent SSD detection for M.2 SATA devices
3) Improve L3$ bandwidth in AIDA64
4) Improve stability if user disables cores on 5600X/5800X with AMD Ryzen Master pic.twitter.com/KqGu78rqiE— AMD Ryzen (@AMDRyzen) February 25, 2021
According to other sources, the AGESA 1.2.0.1 BIOS Firmware will also address the L3 cache performance issue that users were facing when updating to 1.2.0.0 BIOS Firmware. Reports indicate that a test was made to check the claims, and an AMD Ryzen 9 5950X CPU and an MSI MEG B550 Unify motherboard were used. The comparison was done with both the AGESA 1.2.0.0 and the new AGESA 1.2.0.1 firmware.
With older version AGESA 1.2.0.0, the L3 cache bandwidth was severely limited on certain boards. As you can see in the below screenshot, the L3 read and write bandwidth were just under 300GB/s and 350GB/s, respectively with the older firmware:
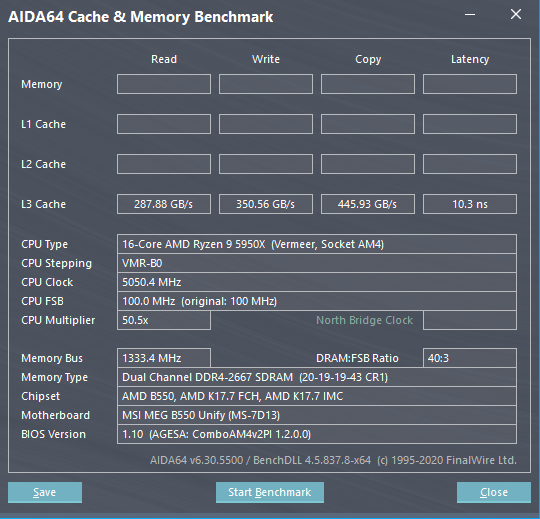
With the update, the same are pushed to over 1,000GB/s and 848GB/s, respectively, an increase of around 3x. It’s unclear whether every processor was affected by this bus and how much of an impact did it have on performance across various workloads, but there should certainly be a notifiable uplift in many applications.
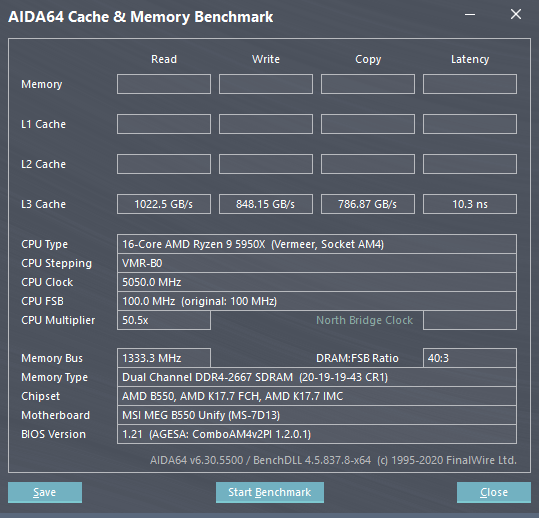
The AGESA 1.2.0.1 fixes the L3 cache performance issues and the numbers are back to normal where they should be. Also, MSI has initially rolled out the AMD AGESA 1.2.0.1 Firmware on the following 500-series motherboards:
- MEG X570 GODLIKE (7C34.v1D1 Beta)
- MEG X570 ACE (7C35.v1E1 Beta)
- MEG X570 Unify (7C35.vA91 Beta)
- MEG B550 Unify (7D13.v121 Beta)
- MEG B550 Unify-X (7D13.vA21 Beta)
If you are running any of these motherboards, make sure to get the latest BIOS from MSI’s to unlock the full potential of your AMD Ryzen 500 series desktop processor. We will keep you updated when other manufacturers release their respective BIOS Firmware based on the AMD AGESA 1.2.0.1 version.