
The same way that the Snapdragon 870 is an overclocked 865+, the 860 too is an overclocked 855+. Rebranding of older chips with new names is Qualcomm’s way of reusing an older node on a flagship 8xx chip numbering scheme. Qualcomm eventually released the 860 today packed deep inside the Poco X3 Pro! The million dollar question is, why would Qualcomm rebrand older chips especially one that is 2 years old? The reason is… LG recently launched the LG Velvet on the Snapdragon 845 node, one from 3 years ago. But there was a shortage of Snapdragon 845 devices, so Qualcomm decided to rebrand existing 2019 flagships. Qualcomm openly embracing this strategy and repurposing their older silicon for cheaper devices is definitely a step in the right direction. Snapdragon 860-powered smartphones will perform just as good, if not better, than 2019 flagships and will also be more powerful than a good chunk of current mid-range devices.
The 860 sticks to the same old 7nm process node
The Snapdragon 855, 855+, 860, 865, 865+ and the 870 are all fabricated on the 7nm process node. Qualcomm is making good use of the 7nm process node due to stocks shortage of the 5nm process node which also results in a shortage of the Snapdragon 888. The good thing about the 860 and the 870 is a higher binned version of the 7nm node which allows Qualcomm to overclock the first core higher. Thus the 860 and 870 have higher clocks as compared to their counterparts 855+ and 865+. The only drawback of the 860 and 870 is the lack of 5G support. Fortunately, the 860 still supports 16GB RAM on the LPDDR4 standard. So 16/512GB phone variants will be easier to manufacture.

Poco X3 Pro
Poco Global has officially announced the Poco X3 Pro. It is an all-new phone and a successor to PocoX3 NFC. Last year, Poco X3 NFC was introduced and it became very popular thanks to the features like 120Hz refresh rate display, Snapdragon 732G, and more. With the Poco X3 Pro, the company has improved the performance even more. Let’s check out the Poco X3 Pro in detail below.
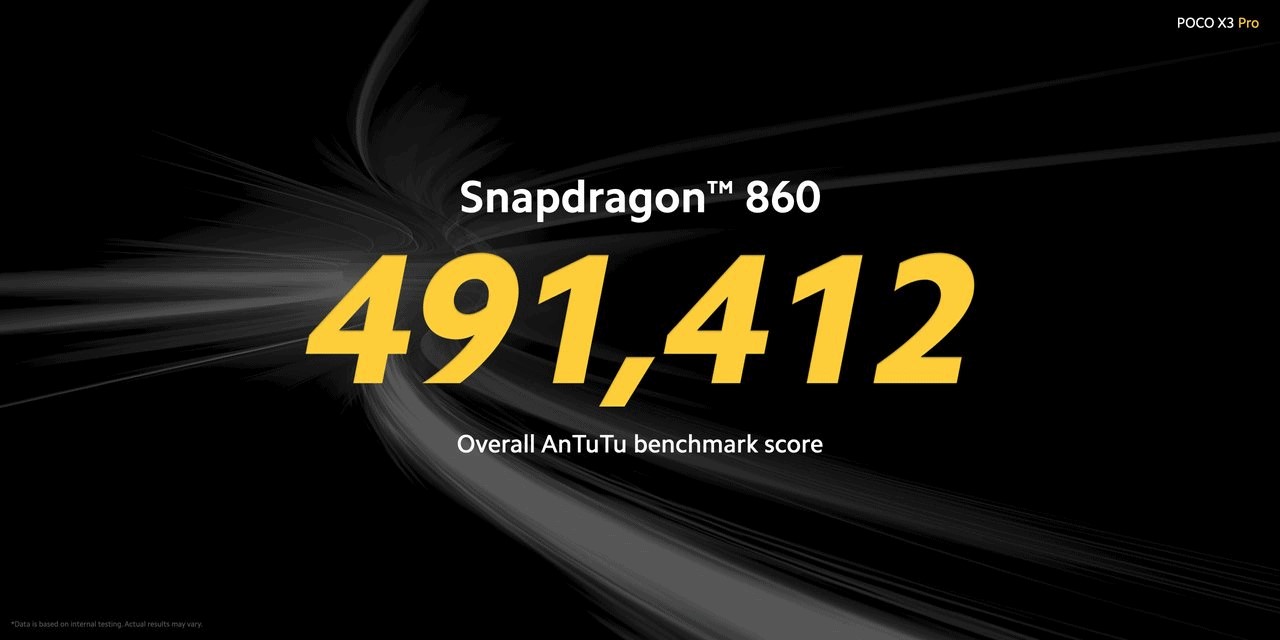
Performance
As stated earlier, the Poco X3 Pro comes with the latest Qualcomm Snapdragon 860, the 7nm fabricated chipset and it sits between the Qualcomm Snapdragon 855+ and 860 processors. The chipset uses the Adreno 640 GPU. According to the company, Poco X3 Pro was able to get an average of 50 frames per second in Genshin Impact on high settings. It is quite good considering Genshin Impact is a graphics intense game. The phone also has a faster UFS 3.1 storage and up to 8GB RAM, unlike Poco X3 NFC which was limited to 6GB RAM. The phone also has an expandable storage option of up to 1TB. The phone has a 5160mAH battery with 33W fast charging.

Camera
Moving forward, the Poco X3 Pro has a quad-camera setup comprising of a 48MP Sony IMX582 primary sensor, 8MP ultra-wide, 2MP macro, and 2MP depth sensors. The camera has many features thanks to the flagship 800-series chipset. It features an Ultra-wide night mode, Video Clones, Dual video, and more. The phone has a 20MP front-facing camera. The phone also has a Night Mode selfie feature.

Display & Design
On the front, Poco X3 Pro features a 6.67-inch IPS LCD panel with a 120Hz refresh rate and 240Hz touch sampling rate. The Poco X3 Pro The phone has a special feature called DynamicSwitch which switches between 60, 90 and 120Hz automatically, without the need for manually choosing the desired refresh rate. There’s Corning Gorilla Glass 6 protection on the front as well. The phone keeps a similar design to last year’s X3 NFC. Although, there are minor changes. This time it is a matte finish thanks to the dual texture body back. The phone also features anti-fingerprint sides. The phone keeps the dual stereo speakers from last year. Although this time, it gets certified from DxOMark audio with 63 points.
Other features
Just like the POCO X3, the ‘Pro’ variant too comes with the 3.5mm audio jack. There is also dual-speakers with Hi-Res Audio certified. As per the DXOMARK Audio review, it gets an overall score of 63 points. Apart from this, the device also has a z-axis linear vibration motor for haptics, NFC, as well as IR Blaster.
Pricing and variants
Finally, coming to the pricing and variants, the Poco X3 Pro comes in two variants. The 6/128GB variant comes at the price tag of 249 Euros (~$297), and the early bird price of this variant is 199 Euros (~$237). While the 8/256GB variant comes at a price tag of 299 Euros (~$356) and the early bird price is 249 Euros (~$297). The phone comes in three colors including Phantom black, frost blue, and metal bronze.
Are you excited for the Poco X3 pro? Let us know your opinions in the comments section below!





