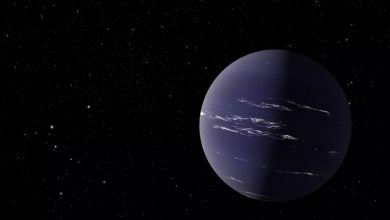
Lead Image: Studying a material that even more closely resembles the composition of ice giants, researchers found that oxygen boosts the formation of diamond rain. The team also found evidence that, in combination with the diamonds, a recently discovered phase of water, often described as “hot, black ice” could form. Credit: Greg Stewart/SLAC National Accelerator Laboratory
SLAC scientists found that oxygen boosts this exotic precipitation, revealing a new path to make nanodiamonds here on Earth.
According to new research, “diamond rain,” a long-hypothesized exotic type of precipitation on ice giant planets, could be more common than previously thought.
In a previous experiment, scientists mimicked the extreme temperatures and pressures found deep inside ice giants Neptune and Uranus and, for the first time, observed diamond rain as it formed.
Investigating this process in a new material that more closely resembles the chemical makeup of Neptune and Uranus, scientists discovered that the presence of oxygen makes diamond formation more likely. This means they are able to form and grow at a wider range of conditions and throughout more planets.
The new study, by researchers from the Department of Energy’s SLAC National Accelerator Laboratory and their colleagues, provides a more complete picture of how diamond rain forms on other planets. Here on Earth, the findings could lead to a new way of fabricating nanodiamonds, which have a very wide array of applications in drug delivery, noninvasive surgery, medical sensors, sustainable manufacturing, and quantum electronics.
“The earlier paper was the first time that we directly saw diamond formation from any mixtures,” Siegfried Glenzer said. He is director of the High Energy Density Division at SLAC. “Since then, there have been quite a lot of experiments with different pure materials. But inside planets, it’s much more complicated; there are a lot more chemicals in the mix. And so, what we wanted to figure out here was what sort of effect these additional chemicals have.”
The team, led by the Helmholtz-Zentrum Dresden-Rossendorf (HZDR) and the University of Rostock in Germany, as well as France’s École Polytechnique in collaboration with SLAC, published the results today (September 2, 2022) in Science Advances.
Starting with plastic
In the earlier experiment, the researchers studied a plastic material made from a mixture of hydrogen and carbon. These are key components of the overall chemical composition of Neptune and Uranus. But in addition to carbon and hydrogen, ice giants contain other elements, such as large amounts of oxygen.
In the more recent experiment, the scientists used PET plastic – often used in food packaging, plastic bottles, and containers – to reproduce the composition of these planets more accurately.
“PET has a good balance between carbon, hydrogen, and oxygen to simulate the activity in ice planets,” Dominik Kraus said. He is a physicist at HZDR and professor at the University of Rostock.
Oxygen is a diamond’s best friend
The scientists used a high-powered optical laser at the Matter in Extreme Conditions (MEC) instrument at SLAC’s Linac Coherent Light Source (LCLS) to create shock waves in the PET. Then, they used X-ray pulses from LCLS to probe what happened in the plastic.
Using a technique called X-ray diffraction, they watched as the atoms of the material rearranged into small diamond regions. They simultaneously used another method called small-angle scattering, which had not been used in the first experiment, to measure how fast and large those regions grew. Using this additional technique, they were able to determine that these diamond regions grew up to a few nanometers wide. They discovered that, with the presence of oxygen in the material, the nanodiamonds were able to grow at lower pressures and temperatures than previously observed.
“The effect of the oxygen was to accelerate the splitting of the carbon and hydrogen and thus encourage the formation of nanodiamonds,” Kraus said. “It meant the carbon atoms could combine more easily and form diamonds.”
Iced-out planets
The team predicts that diamonds on Neptune and Uranus would become much larger than the nanodiamonds produced in these experiments – maybe millions of carats in weight. Over thousands of years, the diamonds might slowly sink through the planets’ ice layers and collect into a thick layer of bling around the solid planetary core.
The scientists also found evidence that, in combination with the diamonds, superionic water might also form. This recently discovered phase of water, often described as “hot, black ice,” exists at extremely high temperatures and pressures. In these extreme conditions, water molecules break apart and oxygen atoms form a crystal lattice in which the hydrogen nuclei float around freely. Since these free-floating nuclei are electrically charged, superionic water can conduct electric current and could explain the unusual magnetic fields on Uranus and Neptune.
The findings could also impact our understanding of planets in distant galaxies, because researchers now believe ice giants are the most common form of planet outside our solar system.
“We know that Earth’s core is predominantly made of iron, but many experiments are still investigating how the presence of lighter elements can change the conditions of melting and phase transitions,” said SLAC scientist and collaborator Silvia Pandolfi. “Our experiment demonstrates how these elements can change the conditions in which diamonds are forming on ice giants. If we want to accurately model planets, then we need to get as close as we can to the actual composition of the planetary interior.”
Diamonds in the rough
The research also indicates a potential path forward for producing nanodiamonds by laser-driven shock compression of cheap PET plastics. While already included in abrasives and polishing agents, in the future, these tiny gems could potentially be used for quantum sensors, medical contrast agents, and reaction accelerators for renewable energy.
“The way nanodiamonds are currently made is by taking a bunch of carbon or diamond and blowing it up with explosives,” said SLAC scientist and collaborator Benjamin Ofori-Okai. “This creates nanodiamonds of various sizes and shapes and is hard to control. What we’re seeing in this experiment is a different reactivity of the same species under high temperature and pressure. In some cases, the diamonds seem to be forming faster than others, which suggests that the presence of these other chemicals can speed up this process. Laser production could offer a cleaner and more easily controlled method to produce nanodiamonds. If we can design ways to change some things about the reactivity, we can change how quickly they form and therefore how big they get.”
Next, the investigators are planning similar experiments using liquid samples containing ethanol, water, and ammonia – what Uranus and Neptune are mostly made of – which will bring them even closer to understanding exactly how diamond rain forms on other planets.
“The fact that we can recreate these extreme conditions to see how these processes play out on very fast, very small scales is exciting,” said SLAC scientist and collaborator Nicholas Hartley. “Adding oxygen brings us closer than ever to seeing the full picture of these planetary processes, but there’s still more work to be done. It’s a step on the road towards getting the most realistic mixture and seeing how these materials truly behave on other planets.”
Reference: “Diamond formation kinetics in shock-compressed C-H-O samples recorded by small-angle X-ray scattering and X-ray diffraction” 2 September 2022, Science Advances.
DOI: 10.1126/sciadv.abo0617
The research was supported by DOE’s Office of Science and the National Nuclear Security Administration. LCLS is a DOE Office of Science user facility.
Parts of this article have been adapted from an article written by Helmholtz-Zentrum Dresden-Rossendorf.