We found applications that will help to achieve a good figure without leaving home.
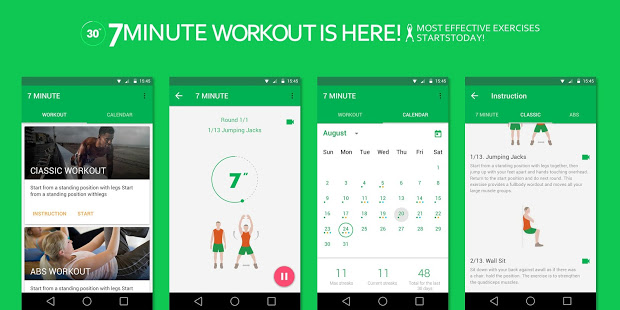
7 Minute Workout
If the treadmill is deadlocked, this is no reason to wait for the appearance of excess weight. Even if you give simple exercises a few minutes a day, it will give a more noticeable result than idleness. Gather yourself together and put laziness out the door will help enterprising application Seven. Running it, you will hear a whistle, and you will have nothing left but for seven minutes to repeat the movements depicted in the pictures. Each exercise is given 30 seconds, and there is a 15-second break between sets.
Pluses
- During each break the app warns you which exercises are coming next.
- All exercises are accompanied by the sounds of the beginning and end of the approaches and other comments. This way you can put your device aside and focus on physical suffering.
- The coaches voices can be changed.
- Almost all exercises are focused on the complete absence of sports equipment. In most cases, you will only need a floor and a wall. So do not rush to throw them out!
- If you get a taste, keep in mind that all exercises can be repeated countless times.
- Seven has a system of awards, and the most sports users will be nicknamed “athlete”.
Minuses
- The pictures do not show for which muscle group the exercise is intended, so the next morning may get sick is not what you are hard rocked the day before.
- Do not indicate the number of repetitions and intensity of exercises.
- Any exercise can be skipped, immediately moving to the very last.
- Some exercises are not described in great detail.
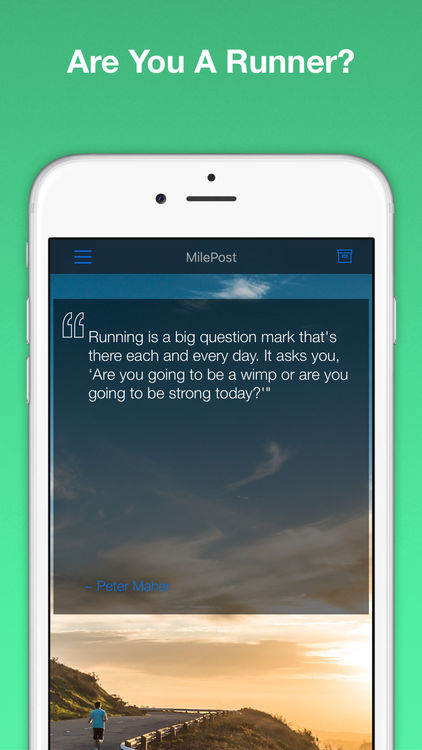
MilePost
The motivating application for latent joggers every morning unsuccessfully trying to unstick yourself from the couch and get out of the closet running shoes. In fact, MilePost does not offer revolutionary solutions — all its functionality is daily throwing on the screen push-notifications with inspiring quotes on the topic of running and sports in General. Those who manage to motivate themselves to run, in this case it is best to rely on Nike+ Running, RunKeeper or Zombies, Run!.
Pluses
- The authorship of all quotations is indicated, and their authenticity can be verified by a direct link to Wikipedia.
- In addition to inspiration quotes in MilePost can be set a reminder of the upcoming training.
Minuses
- For disabling annoying ads that have nothing to do with inspiration and motivation, you will have to pay.
- All previous quotes cannot be viewed.
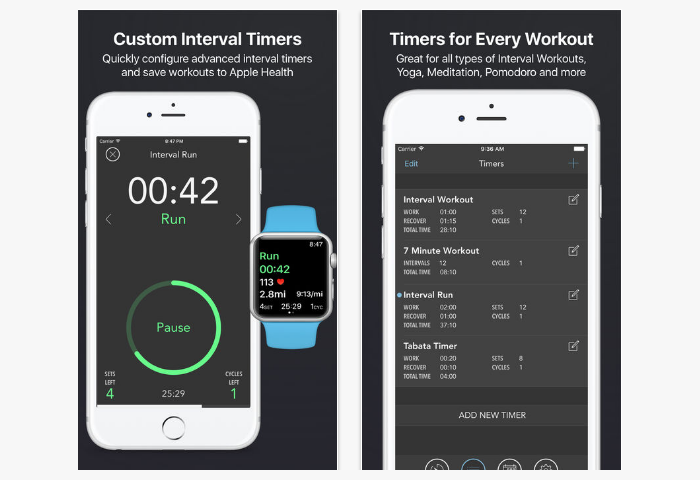
Fitness Intervals
The perfect app for the disorganized and forgetful people who a couple of seconds after the start of charging accidentally switch to sleep or watching TV series. Not to forget, when you need to take the following approach push-ups, and when to rest, you need to start charging Fitness Intervals. With this application, you can keep track of the number of approaches and time spent on exercises, and even set a sound timer with the exact number of approaches, periods of exercise and rest.
If the Fitness Intervals does not meet expectations, you can try to switch to its equally cute competitor Bit Timer.
Pluses
- The duration of rest and exercise periods can be easily adjusted.
- The application has several types of countdown: stopwatch, timer and TABATA.
- The design is both beautiful and functional.
Minuses
- Very quiet and unintelligible sounds of the end / beginning of periods.
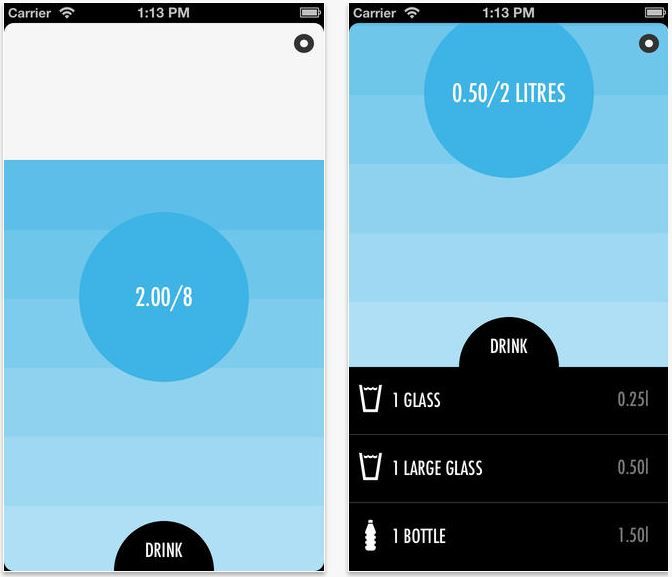
WaterIn
During sports, there is often a desire to overturn a gallon of water inside. Not to drink too much will help minimalist, but very useful application Water in, which helps to monitor the daily rate of water consumption. To do this, you only need to mark the amount of liquid you drink during the day.
Pluses
- You can mark the dose of drunk: a small glass, a large glass, a bottle.
- If you are used to drinking from non-standard containers, the liter can be set manually.
- For those who regularly forget to drink, notifications are provided.
Minuses
- All you drink per day to reset and drink the same. No one will punish you for that.
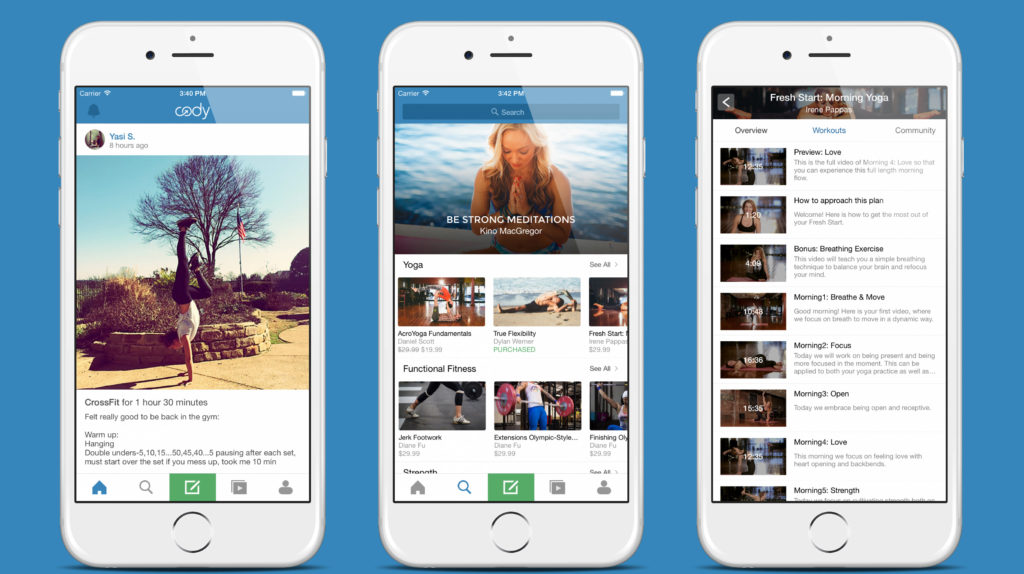
Cody
The main disappointment of the beginners is that none of the others will not see what you’re doing until your costume in public will not burst under the pressure of the muscles. Fortunately, there are specialized social networks for such cases. For example, in Cody you can post photos of your sports feats and sufferings without a twinge of conscience and change statuses with the speed of a minute hand. In addition, here you can subscribe to well-known coaches and other masters of sports to always be aware of sports life hacking. After all, if you don’t belong to the sports people breed, Cody can be used to observe the suffering of others.
Pluses
- Before you start using, you will be prompted to find friends from Facebook. Make no mistake: they are not here anyway.
- Likes and comments to your posts motivate better than the most brutal coaches. Most importantly, to have someone to put.
- News feed Cody is divided into types of sports Hobbies users: yoga, running, Cycling, walking and the like. This allows you to keep track of only the most relevant updates.
- In the Training section you can subscribe to sports programs from professional athletes, but most of them cost money.
- In the calendar you can describe in detail all the exercises, and your friends (if any) will see it.
Minuses
- App designed exclusively for the iPhone.
- Quite confusing to navigate.
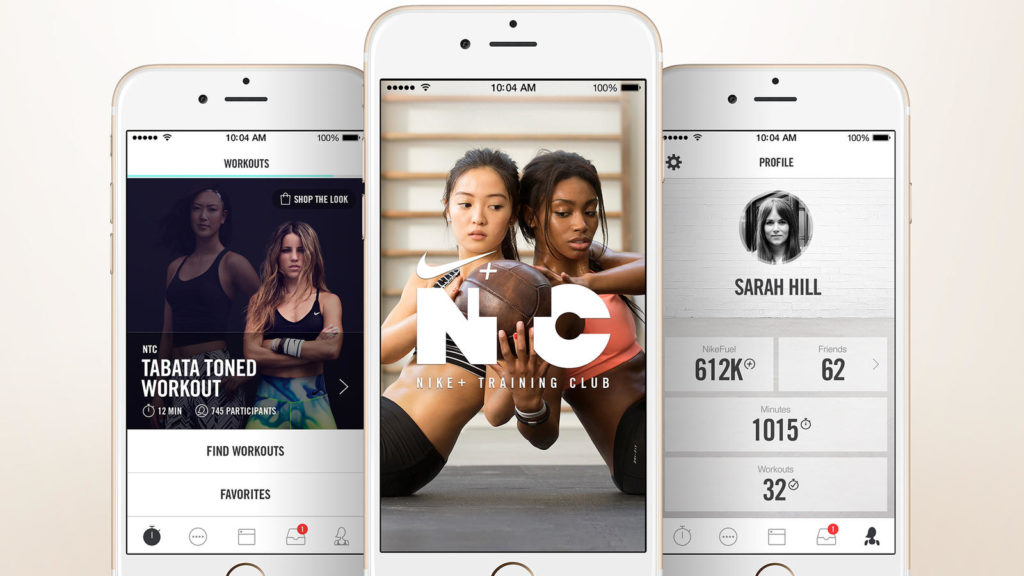
Nike Training Club
This app is deservedly included in our list of the most useful apps of 2019. The fact that it is worth paying attention to, says at least one fact: Nike is focused exclusively on the female half of the sports population of the Earth, but despite this, it is successfully used by many men.
Teemo
It is much easier to start doing sports than to continue. In order not to fall on the first squat, you can try to deceive yourself with the application Teemo, cleverly disguised as a fun game. Its idea is quite simple: choosing a place of action (Wild West, jungle, Everest, and so on), you need, as in a normal Board game, move along a given route, performing parallel strength exercises. However, for a long time to be in the dark you will not work: falling dead after a few tasks, you will realize that this is not a game, but a real sports training with all the ensuing pain in the muscles.
Pluses
- There is a choice of difficulty level.
- In case of acute attacks of loneliness, you can always invite up to nine friends to the team.
- All exercises are accompanied by video instructions performed by cute athletes.
Minuses
- The developers did not consider it necessary to optimize their application for iPad, so it will look beautiful only on the iPhone.
- Unfortunately, there is no Big Brother — no one will monitor the correct implementation of the exercises.
Workout Trainer
To decide to go in for sports is already a feat worthy of respect. Those who do not find the strength to stop in time, can use the services of Workout Trainer. The developers promise more than a thousand different exercises with detailed descriptions and video instructions. To use this application to amass a pain in all the muscles, simultaneously becoming strong and hardy, you only need to choose the level of difficulty, the goal, the desired duration of training and begin to perform the exercises shown in the pictures.
Pluses
- In addition to view instructions, all exercises are accompanied by a voice description that allows you to engage without looking at the screen.
- All video instructions are shot no worse than in the same Nike Training Club.
- There is an opportunity to choose coaches. However, the free version is available only cartoon ladies with robotic voices.
Minuses
- A large number of menu items and other details leads to instant disorientation and the desire to quickly turn off the application.
- You must be connected to the Internet to view some content.
- To get access to all training sessions and videos in high resolution, you will have to pay for a PRO account.
- Many athletes in the video do not look very sporty and attractive. In the same Nike Training Club to perform exercises you are taught by real superhumans with perfect bodies.