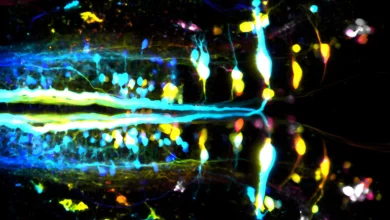
Lead Image: The complete set of neurons in an insect brain, which were reconstructed using synapse-resolution electron microscopy. Credit: Johns Hopkins University/University of Cambridge
In the quest to understand how we think, “everything has been working up to this.”
Researchers have completed the most advanced brain map to date, that of an insect, a landmark achievement in neuroscience that brings scientists closer to true understanding of the mechanism of thought.
“It’s been 50 years and this is the first brain connectome. It’s a flag in the sand that we can do this.” — Joshua T. Vogelstein, Associate professor, Whiting School of Engineering
The international team led by Johns Hopkins University and the University of Cambridge produced a breathtakingly detailed diagram tracing every neural connection in the brain of a larval fruit fly, an archetypal scientific model with brains comparable to humans.
The work, likely to underpin future brain research and to inspire new machine learning architectures, appears today (March 10, 2023) in the journal Science.
“If we want to understand who we are and how we think, part of that is understanding the mechanism of thought,” said senior author Joshua T. Vogelstein, a Johns Hopkins biomedical engineer who specializes in data-driven projects including connectomics, the study of nervous system connections. “And the key to that is knowing how neurons connect with each other.”
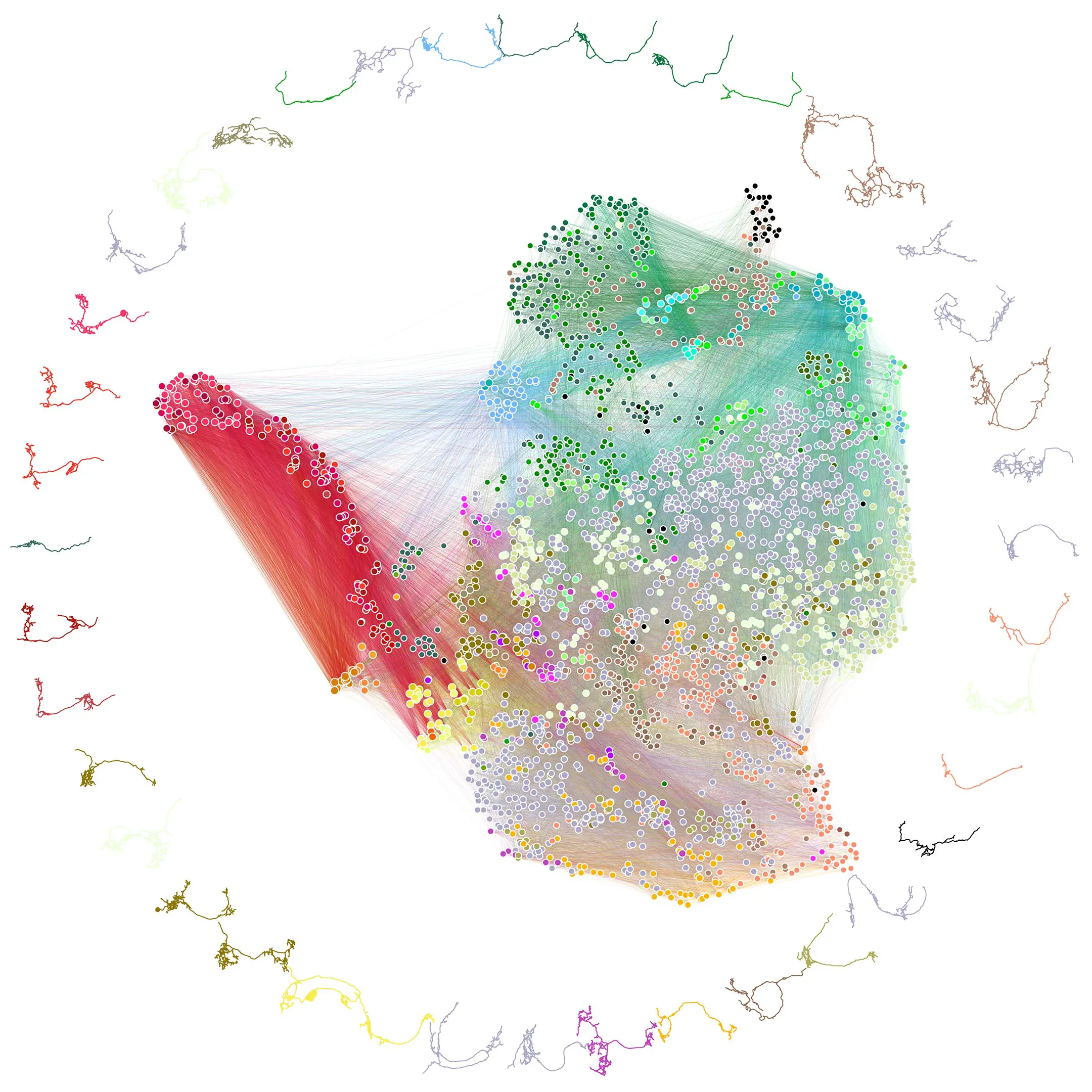
The first attempt at mapping a brain—a 14-year study of the roundworm begun in the 1970s, resulted in a partial map and a Nobel Prize. Since then, partial connectomes have been mapped in many systems, including flies, mice, and even humans, but these reconstructions typically only represent only a tiny fraction of the total brain. Comprehensive connectomes have only been generated for several small species with a few hundred to a few thousand neurons in their bodies–a roundworm, a larval sea squirt, and a larval marine annelid worm.
This video moves through cross-sections of the brain to reveal the final reconstructed neurons. Credit: Johns Hopkins University/University of Cambridge
This team’s connectome of a baby fruit fly, Drosophila melanogaster larva, is the most complete as well as the most expansive map of an entire insect brain ever completed. It includes 3,016 neurons and every connection between them: 548,000.
“It’s been 50 years and this is the first brain connectome. It’s a flag in the sand that we can do this,” Vogelstein said. “Everything has been working up to this.”
Mapping whole brains is difficult and extremely time-consuming, even with the best modern technology. Getting a complete cellular-level picture of a brain requires slicing the brain into hundreds or thousands of individual tissue samples, all of which have to be imaged with electron microscopes before the painstaking process of reconstructing all those pieces, neuron by neuron, into a full, accurate portrait of a brain. It took more than a decade to do that with the baby fruit fly. The brain of a mouse is estimated to be a million times larger than that of a baby fruit fly, meaning the chance of mapping anything close to a human brain isn’t likely in the near future, maybe not even in our lifetimes.
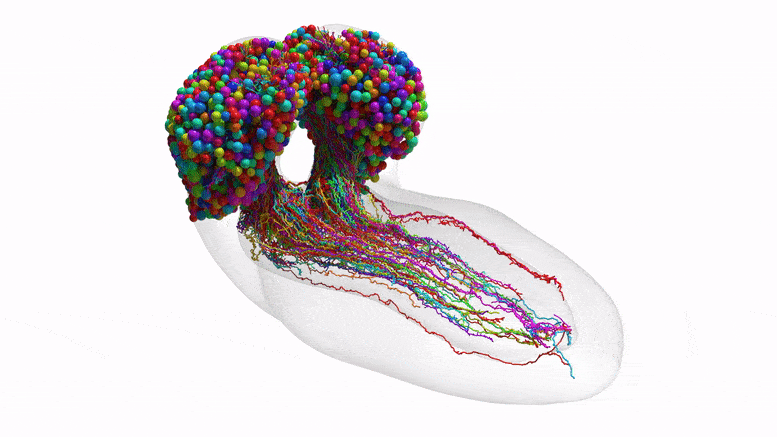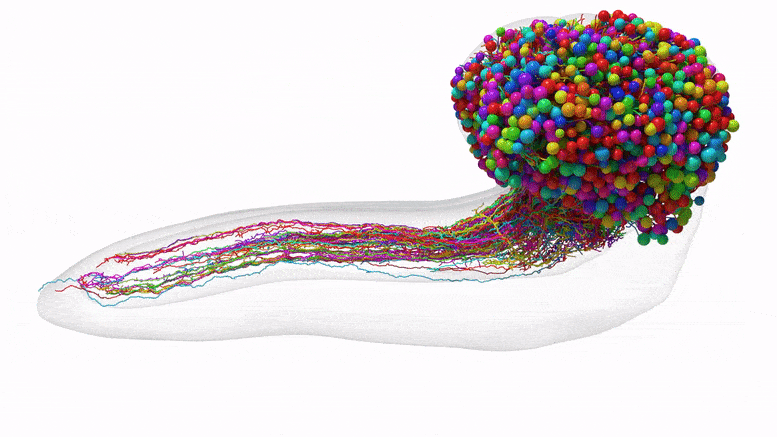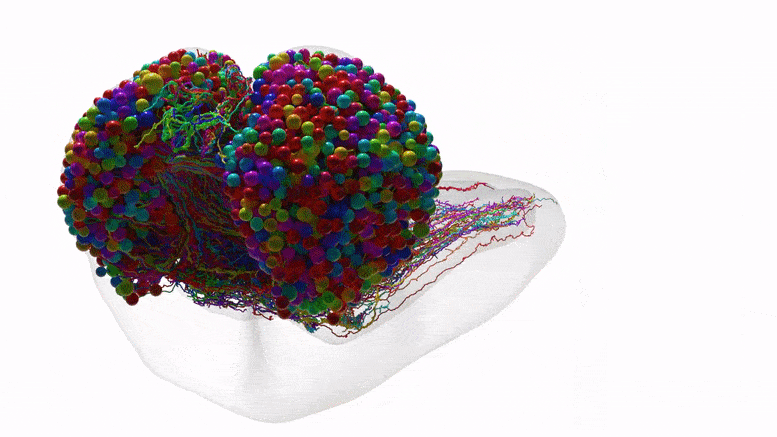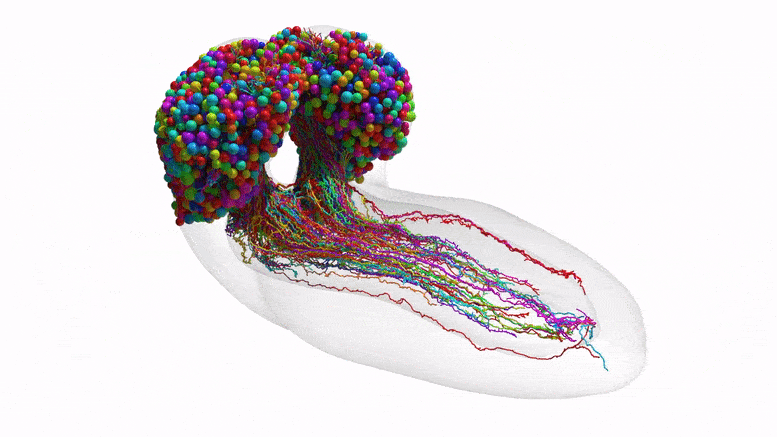
The team purposely chose the fruit fly larva because, for an insect, the species shares much of its fundamental biology with humans, including a comparable genetic foundation. It also has rich learning and decision-making behaviors, making it a useful model organism in neuroscience. And for practical purposes, its relatively compact brain can be imaged and its circuits reconstructed within a reasonable time frame.
Even so, the work took the University of Cambridge and Johns Hopkins 12 years. The imaging alone took about a day per neuron.
Cambridge researchers created the high-resolution images of the brain and manually studied them to find individual neurons, rigorously tracing each one and linking their synaptic connections.
Cambridge handed off the data to Johns Hopkins, where the team spent more than three years using original code they created to analyze the brain’s connectivity. The Johns Hopkins team developed techniques to find groups of neurons based on shared connectivity patterns, and then analyzed how information could propagate through the brain.
In the end, the full team charted every neuron and every connection, and categorized each neuron by the role it plays in the brain. They found that the brain’s busiest circuits were those that led to and away from neurons of the learning center.
The connectome depicts how neurons communicate within each brain hemisphere and across brain hemispheres. Credit: Johns Hopkins University/University of Cambridge
The methods Johns Hopkins developed are applicable to any brain connection project, and their code is available to whoever attempts to map an even larger animal brain, Vogelstein said, adding that despite the challenges, scientists are expected to take on the mouse, possibly within the next decade. Other teams are already working on a map of the adult fruit fly brain. Co-first author Benjamin Pedigo, a Johns Hopkins doctoral candidate in Biomedical Engineering, expects the team’s code could help reveal important comparisons between connections in the adult and larval brain. As connectomes are generated for more larva and from other related species, Pedigo expects their analysis techniques could lead to better understanding of variations in brain wiring.
The fruit fly larva work showed circuit features that were strikingly reminiscent of prominent and powerful machine learning architectures. The team expects continued study will reveal even more computational principles and potentially inspire new artificial intelligence systems.
“What we learned about code for fruit flies will have implications for the code for humans,” Vogelstein said. “That’s what we want to understand—how to write a program that leads to a human brain network.”
Reference: “The connectome of an insect brain” by Michael Winding, Benjamin D. Pedigo, Christopher L. Barnes, Heather G. Patsolic, Youngser Park, Tom Kazimiers, Akira Fushiki, Ingrid V. Andrade, Avinash Khandelwal, Javier Valdes-Aleman, Feng Li, Nadine Randel, Elizabeth Barsotti, Ana Correia, Richard D. Fetter, Volker Hartenstein, Carey E. Priebe, Joshua T. Vogelstein, Albert Cardona and Marta Zlatic, 10 March 2023, Science.
DOI: 10.1126/science.add9330
Authors included: Michael Winding, Christopher L. Barnes, Heather G. Patsolic, Youngser Park, Tom Kazimiers, Akira Fushiki, Ingrid V. Andrade, Avinash Khandelwal, Javier Valdes-Aleman, Feng Li, Nadine Randel, Elizabeth Barsotti, Ana Correia, Richard D. Fetter, Volker Hartenstein, Carey E. Priebe, Albert Cardona, and Marta Zlatic.
Funding: Howard Hughes Medical Institute, Wellcome Trust, Wellcome Trust, NIH/National Institutes of Health, Defense Advanced Research Projects Agency, Air Force Research Laboratory, NIH/National Institutes of Health, National Science Foundation, National Science Foundation