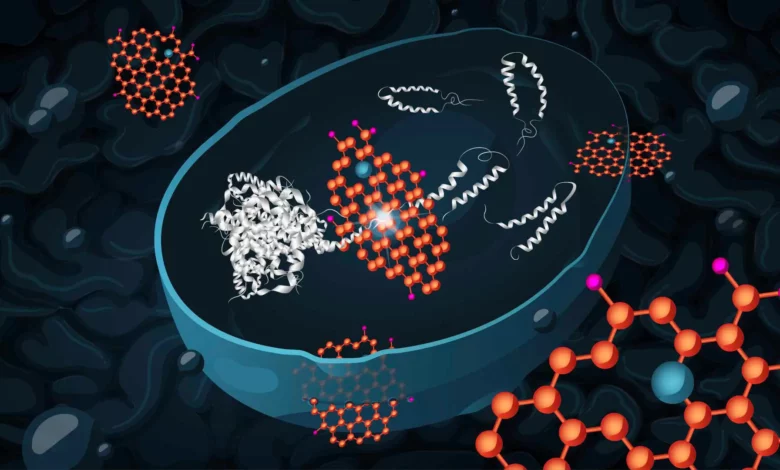
Lead Image: Graphene oxide (orange) can effectively enter yeast cells and reduce the toxicity of harmful protein aggregates (light grey), by promoting disassembly and then degradation of the aggregates. Researchers at Chalmers University of Technology have developed a yeast model, which mimics the neurons in a human brain affected by Alzheimer’s disease, to demonstrate this. Moreover (not shown by the illustration), graphene oxide treatment can alter the metabolism of the cells to increase their capacity to cope with stress. Credit: Chalmers University of Technology / Katharina Merl
A probable early driver of Alzheimer’s disease is the accumulation of molecules called amyloid peptides. These cause cell death, and are commonly found in the brains of Alzheimer’s patients. Researchers at Chalmers University of Technology, Sweden, have now shown that yeast cells that accumulate these misfolded amyloid peptides can recover after being treated with graphene oxide nanoflakes.
Alzheimer’s disease is an incurable brain disease, leading to dementia and death, that causes suffering for both the patients and their families. It is estimated that over 40 million people worldwide are living with the disease or a related form of dementia. According to Alzheimer’s News Today, the estimated global cost of these diseases is one percent of the global gross domestic product.
Misfolded amyloid-beta peptides, Aβ peptides, that accumulate and aggregate in the brain, are believed to be the underlying cause of Alzheimer’s disease. They trigger a series of harmful processes in the neurons (brain cells) – causing the loss of many vital cell functions or cell death, and thus a loss of brain function in the affected area. To date, there are no effective strategies to treat amyloid accumulation in the brain.
Researchers at Chalmers University of Technology have now shown that treatment with graphene oxide leads to reduced levels of aggregated amyloid peptides in a yeast cell model.
“This effect of graphene oxide has recently also been shown by other researchers, but not in yeast cells,” says Xin Chen, Researcher in Systems Biology at Chalmers and first author of the study. “Our study also explains the mechanism behind the effect. Graphene oxide affects the metabolism of the cells, in a way that increases their resistance to misfolded proteins and oxidative stress. This has not been previously reported.”
Proteins and Peptides
Proteins and peptides are fundamentally the same type of molecule and are made up of amino acids. Peptide molecules are smaller – typically containing less than 50 amino acids – and have a less complicated structure. Proteins and peptides can both become deformed if they fold in the wrong way during formation in the cell. When many amyloid-beta peptides accumulate in the brain, the aggregates are classified as proteins.
Mechanisms and Experiments
In Alzheimer’s disease, the amyloid aggregates exert their neurotoxic effects by causing various cellular metabolic disorders, such as stress in the endoplasmic reticulum – a major part of the cell, in which many of its proteins are produced. This can reduce cells’ ability to handle misfolded proteins, and consequently increase the accumulation of these proteins.
The aggregates also affect the function of the mitochondria, the cells’ powerhouses. Therefore, the neurons are exposed to increased oxidative stress (reactive molecules called oxygen radicals, which damage other molecules); something to which brain cells are particularly sensitive.
The Chalmers researchers have conducted the study by a combination of protein analysis (proteomics) and follow-up experiments. They have used baker’s yeast, Saccharomyces cerevisiae, as an in vivo model for human cells. Both cell types have very similar systems for controlling protein quality. This yeast cell model was previously established by the research group to mimic human neurons affected by Alzheimer’s disease.
“The yeast cells in our model resemble neurons affected by the accumulation of amyloid-beta42, which is the form of amyloid peptide most prone to aggregate formation,” says Xin Chen. “These cells age faster than normal, show endoplasmic reticulum stress and mitochondrial dysfunction, and have elevated production of harmful reactive oxygen radicals.”
Graphene Oxide Nanoflakes: A Promising Tool
Graphene oxide nanoflakes are two-dimensional carbon nanomaterials with unique properties, including outstanding conductivity and high biocompatibility. They are used extensively in various research projects, including the development of cancer treatments, drug delivery systems, and biosensors.
The nanoflakes are hydrophilic (water soluble) and interact well with biomolecules such as proteins. When graphene oxide enters living cells, it is able to interfere with the self-assembly processes of proteins.
“As a result, it can hinder the formation of protein aggregates and promote the disintegration of existing aggregates,” says Santosh Pandit, Researcher in Systems Biology at Chalmers and co-author of the study. “We believe that the nanoflakes act via two independent pathways to mitigate the toxic effects of amyloid-beta42 in the yeast cells.”
In one pathway, graphene oxide acts directly to prevent amyloid-beta42 accumulation. In the other, graphene oxide acts indirectly by a (currently unknown) mechanism, in which specific genes for stress response are activated. This increases the cell’s ability to handle misfolded proteins and oxidative stress.
Future Perspectives
How to treat Alzheimer’s patients is still a question for the future. However, according to the research group at Chalmers, graphene oxide holds great potential for future research in the field of neurodegenerative diseases. The research group has already been able to show that treatment with graphene oxide also reduces the toxic effects of protein aggregates specific to Huntington’s disease in a yeast model.
“The next step is to investigate whether it is possible to develop a drug delivery system based on graphene oxide for Alzheimer’s disease,” says Xin Chen. “We also want to test whether graphene oxide has beneficial effects in additional models of neurodegenerative diseases, such as Parkinson’s disease.”
Reference: “Graphene Oxide Attenuates Toxicity of Amyloid-β Aggregates in Yeast by Promoting Disassembly and Boosting Cellular Stress Response” by Xin Chen, Santosh Pandit, Lei Shi, Vaishnavi Ravikumar, Julie Bonne Køhler, Ema Svetlicic, Zhejian Cao, Abhroop Garg, Dina Petranovic and Ivan Mijakovic, 07 July 2023, Advanced Functional Materials.
DOI: 10.1002/adfm.202304053
The study has been carried out in the Mijakovic laboratory at the Division of Systems and Synthetic Biology at Chalmers University of Technology, and at Technical University of Denmark (DTU). Authors are Xin Chen, Santosh Pandit, Lei Shi, Zhejian Cao, Dina Petranovic and Ivan Mijakovic at Chalmers, and Vaishnavi Ravikumar, Julie Bonne Køhler, Ema Svetlicic and Abhroop Garg at DTU.
The research has been supported by grants from Vinnova Center CellNova, Novo Nordisk Foundation, Marie Skłodowska-Curie grant, and the Swedish Research Council.