Lead Image: This is an artist’s illustration of a massive, newly forming exoplanet called AB Aurigae b. Researchers used new and archival data from the Hubble Space Telescope and the Subaru Telescope to confirm this protoplanet is forming through an intense and violent process, called disk instability. Disk instability is a top-down approach, much different from the dominant core accretion model. In this scenario, a massive disk around a star cools, and gravity causes the disk to rapidly break up into one or more planet-mass fragments. AB Aurigae b is estimated to be about nine times more massive than Jupiter and orbits its host star over two times farther than Pluto is from our Sun. Credit: NASA, ESA, Joseph Olmsted (STScI)
Hubble Finds a Planet Forming in an Unconventional Way
In general, the formation of planets in our universe can be likened to cooking a meal. Just like the “ingredients” for forming a planet can change, so can the “cooking method.”
Researchers using the Hubble Space Telescope have caught a planet in the act of what could be likened to a “flash fry” — a violent and intense process called disk instability. In this method, instead of having a planet that grows and builds up from a small core accumulating matter and gas, the protoplanetary disk around a star cools, and gravity causes it to break up into one or more planet-mass fragments.
Astronomers have long searched for clear evidence of this process as a viable candidate in forming large, Jupiter-like planets, and Hubble’s resolution and longevity proved to be a key missing puzzle piece.
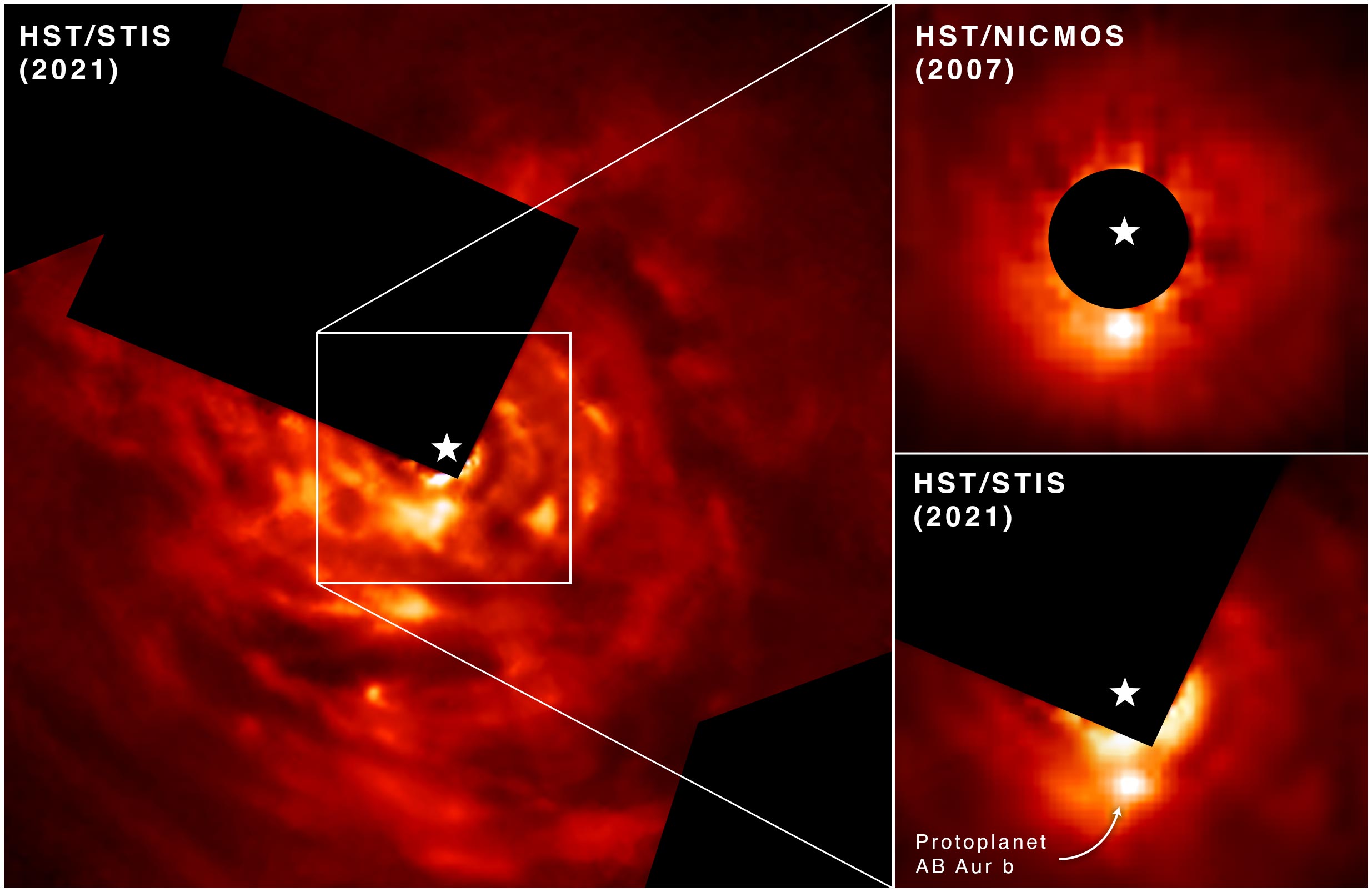
Evidence shows violent collapse responsible for formation of Jupiter-like protoplanet.
NASA’s Hubble Space Telescope has directly photographed evidence of a Jupiter-like protoplanet forming through what researchers describe as an “intense and violent process.” This discovery supports a long-debated theory for how planets like Jupiter form, called “disk instability.”
The new world under construction is embedded in a protoplanetary disk of dust and gas with distinct spiral structure swirling around, surrounding a young star that’s estimated to be around 2 million years old. That’s about the age of our solar system when planet formation was underway. (The solar system’s age is currently 4.6 billion years.)
“Nature is clever; it can produce planets in a range of different ways,” said Thayne Currie of the Subaru Telescope and Eureka Scientific, lead researcher on the study.
All planets are made from material that originated in a circumstellar disk. The dominant theory for jovian planet formation is called “core accretion,” a bottom-up approach where planets embedded in the disk grow from small objects — with sizes ranging from dust grains to boulders — colliding and sticking together as they orbit a star. This core then slowly accumulates gas from the disk. In contrast, the disk instability approach is a top-down model where as a massive disk around a star cools, gravity causes the disk to rapidly break up into one or more planet-mass fragments.
The newly forming planet, called AB Aurigae b, is probably about nine times more massive than Jupiter and orbits its host star at a whopping distance of 8.6 billion miles – over two times farther than Pluto is from our Sun. At that distance it would take a very long time, if ever, for a Jupiter-sized planet to form by core accretion. This leads researchers to conclude that the disk instability has enabled this planet to form at such a great distance. And, it is in a striking contrast to expectations of planet formation by the widely accepted core accretion model.
The new analysis combines data from two Hubble instruments: the Space Telescope Imaging Spectrograph and the Near Infrared Camera and Multi-Object Spectrograph. These data were compared to those from a state-of-the-art planet imaging instrument called SCExAO on Japan’s 8.2-meter Subaru Telescope located at the summit of Mauna Kea, Hawaii. The wealth of data from space and ground-based telescopes proved critical, because distinguishing between infant planets and complex disk features unrelated to planets is very difficult.
“Interpreting this system is extremely challenging,” Currie said. “This is one of the reasons why we needed Hubble for this project—a clean image to better separate the light from the disk and any planet.”
Nature itself also provided a helping hand: the vast disk of dust and gas swirling around the star AB Aurigae is tilted nearly face-on to our view from Earth.
Currie emphasized that Hubble’s longevity played a particular role in helping researchers measure the protoplanet’s orbit. He was originally very skeptical that AB Aurigae b was a planet. The archival data from Hubble, combined with imaging from Subaru, proved to be a turning point in changing his mind.
“We could not detect this motion on the order of a year or two years,” Currie said. “Hubble provided a time baseline, combined with Subaru data, of 13 years, which was sufficient to be able to detect orbital motion.”
“This result leverages ground and space observations and we get to go back in time with Hubble archival observations,” Olivier Guyon of the University of Arizona, Tucson, and Subaru Telescope, Hawaii added. “AB Aurigae b has now been looked at in multiple wavelengths, and a consistent picture has emerged—one that’s very solid.”
The team’s results are published in the April 4, 2022, issue of Nature Astronomy.
“This new discovery is strong evidence that some gas giant planets can form by the disk instability mechanism,” Alan Boss of the Carnegie Institution of Science in Washington, D.C. emphasized. “In the end, gravity is all that counts, as the leftovers of the star-formation process will end up being pulled together by gravity to form planets, one way or the other.”
Understanding the early days of the formation of Jupiter-like planets provides astronomers with more context into the history of our own solar system. This discovery paves the way for future studies of the chemical make-up of protoplanetary disks like AB Aurigae, including with NASA’s James Webb Space Telescope.
Reference: “Images of embedded Jovian planet formation at a wide separation around AB Aurigae” by Thayne Currie, Kellen Lawson, Glenn Schneider, Wladimir Lyra, John Wisniewski, Carol Grady, Olivier Guyon, Motohide Tamura, Takayuki Kotani, Hajime Kawahara, Timothy Brandt, Taichi Uyama, Takayuki Muto, Ruobing Dong, Tomoyuki Kudo, Jun Hashimoto, Misato Fukagawa, Kevin Wagner, Julien Lozi, Jeffrey Chilcote, Taylor Tobin, Tyler Groff, Kimberly Ward-Duong, William Januszewski, Barnaby Norris, Peter Tuthill, Nienke van der Marel, Michael Sitko, Vincent Deo, Sebastien Vievard, Nemanja Jovanovic, Frantz Martinache and Nour Skaf, 4 April 2022, Nature Astronomy.
DOI: 10.1038/s41550-022-01634-x
The Hubble Space Telescope is a project of international cooperation between NASA and ESA (European Space Agency). NASA’s Goddard Space Flight Center in Greenbelt, Maryland, manages the telescope. The Space Telescope Science Institute (STScI) in Baltimore, Maryland, conducts Hubble science operations. STScI is operated for NASA by the Association of Universities for Research in Astronomy, in Washington, D.C.